Particles
2-4
electron
shells, wave-lengths, bonds, others
Copyright © by
Haertel Martin, All Rights Reserved,
mailto mhaertel@naturgesetze.de
This work will continue work up the fundamental particles of the nature
by the newest sight.
It is going out from the theory of plus- minus- original charges
The aim always was absolutely honesty to the nature.
Considerateness to old wrong and doubtful opinion were strictly
forbidden.
The work with the name of ‚particles 2‘ is
the second part of the collected edition 'Particles'
Here
is a link back to Particles 1
Much is presupposed as know.
If you are missing precognitions, there is referred to follow books
respectively documents of the author.
All necessary basis informations to the nature
laws are inside sub-documents of follow chapters respectively books:
Astronomy . . Astrophysics . . Electro . . Doctrine . . Kernel . . Force . . Radiates . . Specials . . Particles
Particles 2 - 4
Content
1a) He-accruement
with a electron
1c) Exteriour electron at Be up to C
1e) p-shell with 4 respectively 5
electrons
2f) End of shell, atoms at the border
negative
1) Energy
and force of weaks in general
1a) Weaks (for ex. light) on basis of
original charges
1b) Necessarity of a separate particle
-energy
1d) Common rules to paricle energy,
difference masse
1e) Intern/extern particle diameter
1f) Intern particle diameter stabil
1g) Basic force effect to plane are and
sphere-surface
1h) Extern particle radius at extern
force variation
1j) Force of shell- and particles nearly
proportional
2) Energy-variation
to distances
2a) E-conservation at strongs --
E-variation at weaks
2b) Distance variation changes the
energy of systems
2c) Summarize of energy behavior of
strongs/weaks
2d) Formeleinsatz zum Energie-Vergleich
2e) Caution at the masse effect
3a) Shells, force tips, filling
3e) Always 2 belong to the acceleration
3f) Acceleration of light in neighbour
shells
3g) Permanent brake and acceleration in
follow shells
3h) Re-capturing respectively c-loss
3j) Shell's force structures very
unprecise
4d) Weaks normally not quantable
4e) Quanting only in chaos or with extrem
separation
5) Quantity
and quality of minos
5b) Atom bond with different shells
respectively waves
5d) Wave-lenths and temperatures
5e) Feature variations at changed
wave-length
1) Gases/atoms
and their external shells
1a) Most narrow E-radius at inert gases
1b) Inert gases have shorter-waved
shells
1c) Inert gases have much more and
smaller shells
1e) Atom to outside positive and
negative at the same time
2d) Basis of the aggregation condition
3b) Melting point and length expanse -
Pb-Ir
3c) Generally to‘only 1 electron
in a shell'
3d) 1 electron outside and full shells
inside - Cu,Ag,Au
3e) With only 1 electron outside very
easy to bind
3f) Effects at only 1 electron in last
shell
3g) More to of effects of 1 or 2
electrons in last shell
3h) Result with only 1 electron in
end-shell
4) Bond
shells of the atom kernel
4a) Common to protons and neutrons
4b) Clearance of protons and neutrons
4c) Extern force reversion of the
nuleon-/atom-kernel
4d) Bond shells of the nucleons
4e) Bond curve of the nucleons
4f) Neutrons are also no gases
4h) Negative Suppe des Nukleonenkerns
5) Proton/Neutron
- welches letzte Elektron ?
5a) Elektronenabgabe und die Bindeschale
5b) Wieviel
Elektronen eines Nukleons in äusserster Schale ?
5c) Differenz
der ersten Schalen bei Neutronen/Proton
5d) Proton
hat eine E-Schale weniger ?
5e) Letzte
E-Schale mit 1 bzw. 2 Elektronen
5f) Schale 1 ohne letzte E-Schale
5h) Massendifferenz von Proton/Neutron
unproblematisch
5i) Schale 1 des Atomkerns als
Bindematerial
6) Kraft
und Aggregatszustand des Kerns – Elektronenaustritt
6a) Kraftdifferenz
von Proton und Neutron weiter weg
6b) Schalenumkehrungen des Neutrons
6d) Aggregatszustand der Nukleonen
6e) Hg - Au und ihre Elektronen
6f) Letztes
Nukleonen-Elektron: Allgemeines zur Schale
6g) Äusserstes Elektron extrem
instabil
6h) Elektronenausklinken mit mittleren
Wellen
7) Force
and mass at nucleon’s border
7a) Second force inversion at
nucleon’s border, proton.
7b) Parameter to the masse of the atom
kernel
7c) Masse at the kernel border
8a) Energy and force proportional
8b) Slipping through barrier layers
8d) Electrons don’t jump between
nucleons
Particles 2
1)
Electron
shells
to 2. .. . to content
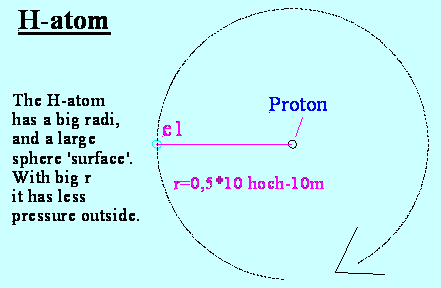
1a) He-accruement with a electron
When at tritium a neutron is delivering one electron
into the electron shell, at once this will rotate at a contra position to the
first, the H-electron.
Now 2 protons (2+) affect to both and with
2-times distance the other electron (1/4*-1). This results a force of +1,75 instead previously 1,0 to the electrons.
Now both electrons reduce their radius and will
be accelerated by the kernel according the radius shorting.
With just under 60% radius both would be
balanced by above calculating.
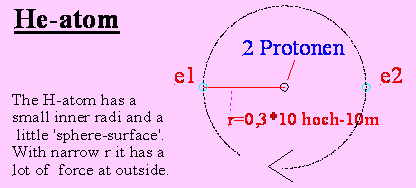
If an electron is leaving a neutron of He, the H- and He-electron will be attracted still norrower
to the kernel (because the kernel has now 3+).
3 electrons cannot rotate within the same
shell.
One of the 3 electrons will be urged far to
outside, the other 2 further to inside.
The most extern electron of these 3 will be
hold by a little bit less than the force 1+, because the both inner electrons
have an average bigger effect to the far away circulating most extern electron
than 2 protons of the kernel.
Therefore the 'Lithium'-electron can have a
bigger radius than the H-electron at hydrogen.
1c)
Exteriour
electron at Be up to C
At leaving of an electron from a neutron from Li
to Beryllium Be it happens principially the same as at the changeover from H to
He. The radius of the electrons is becoming narrower
(at both shells 1s/2s).
At leaving of an electron from a neutron from
Berylium to boron B it happens principially again the same as at the changeover
from He to Li. The electron radius of the new is huge
and a little bit bigger than at Li.
At leaving of an electron from a neutron from
Bor to carbon C it happens again the same like form Li to Be. The electron
radius narrows (at all 3 shells).
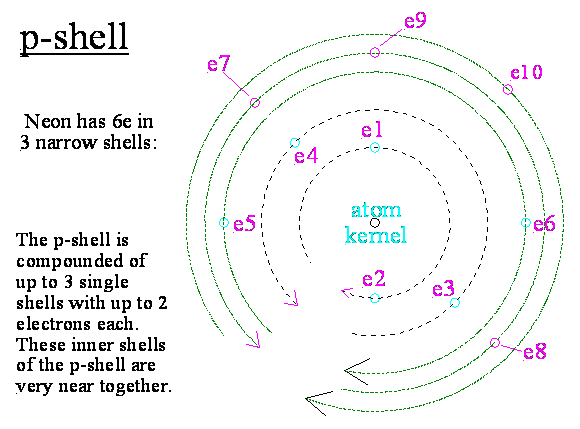
At the changeover from C to N respectively up
to Ne all the additional electrons accumulate wihin the present p-shell.
But the p-shell is intern again compounded of 3
smaller shells with maximal 2 electrons each.
The 3. electron
of the p-shell leaves at once the old opinoin.
It has a little bit bigger radius than the both
inner electons of this shell and is beginning the middle p-shell. Therefore
also melting- and boiling point of phosphorus are so low.
1e)
p-shell
with 4 respectively 5 electrons
The 4. electron
(for ex. N, S) seems to effect similar like the second of the p-shell. It is
attracting the 3. and all others to a narrower radius.
The 5. electron
effects like the 3., but more powerful (for ex. Cl, Br, J).
The 6. electron
effects again like the 4., but still essentially stronger. All are inert gases.
Therefore the p-shell is compounded of 3
shells, in which the electrons are placed in pairs each contrary like within
the s-shells.
All gases have a full exterior p-electron shell
at the atom border (Helium effects also like a full p).
At these gases the last electron contracts all
electron rings essentially more narrow (it electron couple, the first both
inner shells of the p-shell and all farther inside placed electron shells).
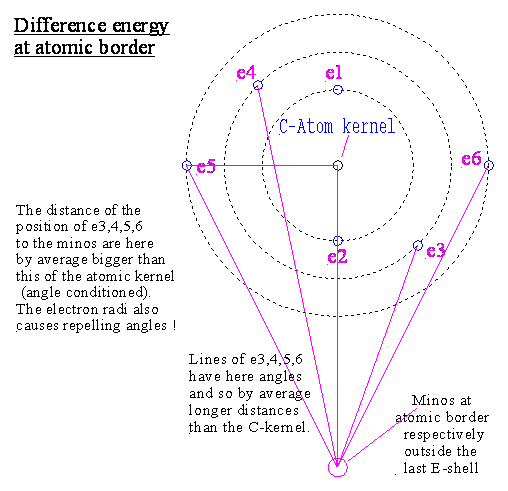
Within the area of the last electron shell we
have a force which holds the last electron.
Direct behind the last electron the force is changing
to the oponent (because of this last electron).
To enough far away situated reference areas the
electrons habe a longer average distance than the protons.
Besides this the electrons have to such
reference areas a force reducing angle because of their radii.
But the strong force of the electrons effects
quadratic to varied distances.
In the anterior part of the atomic sphere, the
electrons have a shorter way to outside points than the protons.
Therefore we can compute by quadratic force a
bigger force of the electrons to outside than from the protons, although the
electrons have overall by average a little bit more distance to outside and a
mutual repelling angle.
Therefore every atom effects
basically to outside with the strong force direction of the electrons.
Now this surplus of force attracts opposite
charged particles.
The 1. force
reversion at the atomic border is directly after the last electron orbit !
2)
Shells at the atom border
to 1. .. . to content .. . beginning
The shells 1a up to 2a are drawed here very
exaggerated wide.
In the reality we find in this wide drawed area
lot of tenths of positive and negative shells.
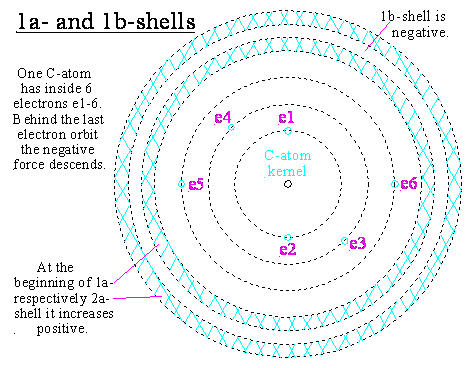
Behind the force reversion 1 the shell 1a has a
force tip.
Behind this the force would descend again
clearly with additional distance.
Shell 1a is attracting with its positive force
negative particles from outside.
These are gathering within the force tip.
With increasing negative energy of this force
tip the positive force descends behind the tip the faster.
From a definite energy on the tip descends
behind it to zero respectively behind that to minus.
At this force reversion 2 shell 1+ (1a) is
ending and the negative shell 1- (1b) is beginning.
The negative shell increases according to the minos energy in shell 1+.
These minos have
opposite to the atomic kernel a short reach of force.
The energy of shell 1- is in all a lot of
smaller than the difference energy from electrons and protons.
Directly aside 1+ the negative force is more
powerful because of the little distance to 1+, behind that it is again
overtrumped by the atomic interior.
With increasing distance from shell 1+ the
negative force is descending quicker than the positive from interior (because
of this different energy quantity and thereby connected reaches of force).
At a definite distance the force of shell 1
becomes null.
The negative shell 1- would attract positive
particles, but is ending too quickly.
Therefore it cannot reach positive particles
outside.
If no positive particles are coming, shell 1-
keeps empty.
At the end of shell 1- we have force reversion
3.
Here shell 2+ is beginning. It is again
positive and attracts from outside present negatives.
Thereby the interior continues attracting
negative particles from far outside.
The powerful particles of these minos are stopping in front of shell 1- (inside 2a). The
more such negative particles are coming the earlier they are building up a
shell 2-.
Negative particles in shell 2a produce again
the reversion shell 2-.
After shell 2+ and 2- still many further shells
can be built up (taking turns with +/-).
At a free atom this could continue so nearly
unendingless.
But it is to observe, that every follow couple
of positive and negative shell is weaker than the preceding.
2f)
End
of shell, atoms at the border negative
It is also to be observed, that the minos energy of shells can surpass the difference energy of
electrons and protons with a sufficient amount.
Then the shell production will end.
Then afterwards the atom is negative up to the
unendlessness.
It also important, that round a positive
original charge can rotate 2 negative. This is the double.
Similarly an inside positive atom can outside
hold a more negative energy than the inside amount of the positive.
This shell system works the same, like one
throws one stone into the water.
Thereby a wave comes into being, which
alternating supplies hill and valley and becomes weaker with additional
distance.
This wave system is at particles like atoms
however 3-dimensional.
With changing surroundings this wave system is
ending. Border more atoms together, this wave system of the nature reaches up
to the waves are bordering these of the neighbour atoms.
2h)
Vibration
of shells ?
The electrons circulate round the atom kernel
and vary therefore permanently the strong difference force between protons and
electrons to outside.
Vibrate therefore all external shells of atoms permanently ?
The farther inside a shell of the same atom is
placed, the higher is their force tip and the force difference of vibration.
Has an atom outside 2 electrons, so the force
difference of vibration is smaller.
The wave length of the shells further inside is
smaller than that of the more extern.
Of that place minos
have the smallest energy and are thereby very indolent.
The circulation of electrons hardly varies
their position.
The further outside the shells are placed, the
more possible variation will be dismantled.
At the atomic bond of more atoms this vibration
is already null.
Particles 3 (wave -lengths)
Energy of weaks - light - quants
Copyright © by
Haertel Martin, All Rights Reserved,
mailto mhaertel@naturgesetze.de
1)
Energy and force of weaks in general
to 2. . . to 3. . . to 4. . . to content
1a)
Weaks
(for ex. light) on basis of original charges
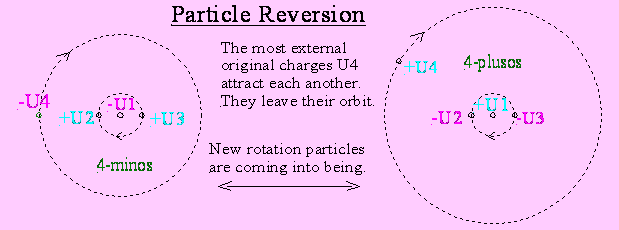
One negative weak (for ex. light-particle) is
compound of 4 original charges, a negative U1- in the middle, 2 near rotating
positive U2+,3+ and one far circulating negative U4-.
These 2 positive and negative original charges
don't neutralize themselves completely, because 3 of these have a radius.
This radius realizes to far outside placed
reference points a greater average distance and a repelling angle.
The force of the paritcle to an exterior
reference point is mostly computed by the radius of -U4 (+rU2 +rU3) and the
distance to this point.
1b)
Necessarity
of a separate particle -energy
The energy (charge energy) of an original charge
is always the same. It is not depending on distances.
By adding the energies of above 4 original
charges, it results 0.
But these 4 original charges (from above
4-particle) have different angles and distances to an extern reference point.
Because that, there is calculated at every other distance and angle another
added force.
The sum of the forces to this point is by
average only 0 at a special ring with an exact radius round this 4-particle.
To outside of this ring we have a force which
it is not zero. To every force also always an energy
is belonging to.
The energy sum of the 4 original charges is
null, but the whole 4-particle has a difference energy to outside, which
depends on the radii of U2,3,4.
Therefore we get a new energy, which may not be
only confound of the pure energy of original charges.
At above 4-particle the -U4 has a powerfuller
force amount to further outside than the sum of -U1, +U2 and +U3 together. The
reason is, -U4 comes much nearer to the reference point and this force is
contrary quadratic to the distances.
The average total force of the 4-particle is
different at every other distance of a reference area.
The energy of above light-particle is computed
of the total force of all 4 original charges at a reference area respectively
of the corresponding sphere surface.
The summarized energy of all 4 original charges
would be null (this E doesn't depend on distances, E=p*m³).
Because of angles and other average distances
of the rotators we get a difference energy from the whole 4-particle to
outside.
This difference energy at an extern sphere
surface is much smaller than the energy amounts of an original charge.
Without changing the intern radius, also this
difference energy of this 4-particle keeps the same.
But this difference energy supplies another
forces at other distances to outside, which are not computed by E=N*m.
Also on the 'sphere surface' of this 4-particle
this force varies not with E=N*m like at original charges.
1d)
Common
rules to paricle energy, difference masse
Above difference energy is the total energy of
above complete particle (light), the particle energy.
This particle energy of 4 original charges is a
fractional amount of the energy of one original charge (for ex. 1/1018).
The same fractional amount of masse of an
original charge hat so much energy like the whole light-particle. We call this
masse fractional amount of an original charge 'difference masse'.
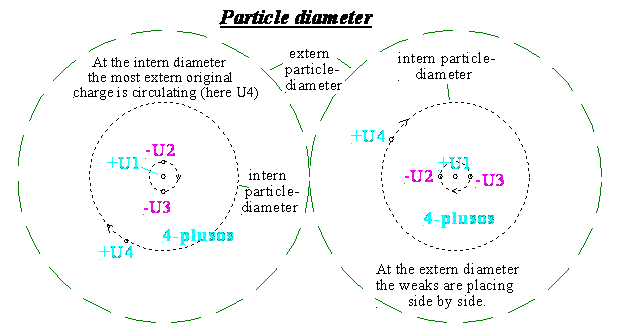
1e)
Intern/extern
particle diameter
The intern particle diameter of a light
particle is at least 2 times more of the radius of its extern original charge
4-.
The extern particle diameter of the light
particle (force diameter) depends on the force of this shell, in which it is
placed.
The intern particle diameter can have 10-40m,
the extern for ex. 10-20m.
1f)
Intern
particle diameter stabil
With varying extern forces the intern particle radius don't changes.
The human being cannot change the intern
particle radius.
People only can separate more particles from
another, but not modify or produce one single of them !
Would the intern particle radius reduce with
pressure from outside, the whole material would collapse within a fractional
amount of a second.
Besides this the difference energy would be
reduced respectively destroyed thereby.
1g)
Basic
force effect to plane are and sphere-surface
At x-times distance
from the center of an original charge the force to a plane area
descends to 1/x².
At x-times distance
from the center of an original charge the force to its own whole sphere
surface descends to 1/x.
2 weaks which have a different energy
amount: the weaker has only within a kind of ellipse-shaped volume a bigger
force effect which surpasses there less weak.
To the rest of the universe the less weak
surpasses the weaker with its force effect.
2 weaks with the same energy amount:
both border extern at an unendlingless plane are. With
2 times distance, the force descends to 1/x4 (m² keeps; N= 1/16
average p * 1m²).
1h)
Extern
particle radius at extern force variation
With x-times distance between the centers of 2
rotation 4-particles (x-times extern particle radius), the force descends to
about 1/x³ up to 1/x4.
With ½ extern particle radius on that
sphere surface the force of a 4-particle increases about to 8-times (N= 32p /
4m²).
But the force of the plane area between two
same 4-particles increases here to 1/x4.
Now it is decisive against which the force is
to calculate, against other equal 4-particles or against the energy of great
shells respectively strong particles.
The pressure of the shell might be at every
point on the sphere surface about 32 times higher, to press light extern to the
halve distances together (32p*1/4m²=8N).
But be careful. With such distance variations
between whole particles the energy of these 4-particles keeps the same.
The energy will be only changed if the intern
particle radius will be changed.
With the 2-times radius of U4 (-U3-U2) the
force of this particle increases about 8- (surface) up to 16-times (plane
area).
Always there is to make difference between the
energy and the force. The energy is 3-dimansional (volume),
the force has a 2-dimensional meter (area).
The same, there must be differenced between the
energy of original charge (cannot be varied) and the energy of weaks (energy
effect is depending on the radii of their original charges).
There where the force does not vary inversely
quadratic to the distances (strong force), you must be careful at calculating
with the factor energy (=difference-energy) !
1j)
Force
of shell- and particles nearly proportional
The force between
equal weaks is changing with x-times distance by 1/x4-times (at the plane area).
Therefore when the shell is varying its force
with factor times 4 or 16, their inside particles will reduce their extern
particle radius nearly to 70,7% respectively 50%.
Then inside the same volume there could be
placed 2,83- respectively 8-times more equal powerful
particles.
The more negative particle energy a positive
shell takes up with arriving minos, the smaller
becomes the reminder positive energy of the shell.
New negative particle reduce the positive
energy of the shell. The shell can take up so much
particles up to be full, respectively their energy to outside is surpassed by
its 4-particles.
Because
above force effect of the weaks (factor 1/x4-times, need of space) also
the force of each particle is important.
More 'little' 4-particles can realize more
energy in the same volume.
When a positive shell is filled up with the
present amount of negative energy, then it is full and cannot take up more
negatives (minos).
Therefore shells with particles of a shorter wave-length (less particle energy) are wider. They need more 'small' particles to fill up
with the same energy amount. But the reversion shell nearly keeps the same bulk
(a little less).
2)
Energy-variation to distances
. . to 1. . . to 3. . . to 4. . . to 5. . . to content . . to the beginning
2a)
E-conservation
at strongs -- E-variation at weaks
The energy behaves at sphere radius
and the sphere surface of original charges
accordingly E=N*m (E keeps; N=p*m²).
At distance
variations x times m to the sphere surface O1 we
get follow parameters: E keeps, O1=*x², p= /x³, N=/x.
At distance
variations x times m between 2 original charges (at a plane
area O2) we get: E keeps, O2 keeps, p= /x², N=/x².
Comparison at 2 times distance at sphere
surface - plane area: p = 1/8 - ¼, O1=x²=4, O2 keeps=1
At distance
variations x times m of a rotation particle to
an extern plane area (2. equal weak) we get: O2
keeps, p= /x4, N=/x4.
At distance
variations x times m of a rotation particle to another particle with
nearly maximal other energy we get: E keeps, O1=*x², p=*1/x5, N=/x³.
You can long examin the consequences of above equations !
Pressure p effects at
distance variations at weaks by 1/x² greater than at strongs.
2b)
Distance
variation changes the energy of systems
If one doesn't varys the intern distance of a
particle, then its energy will not be modified.
Sphere surface of a strong: Here the energy is accordingly E = N * m. The
energy is at every m (radius variation) the same.
Plane area at strongs: energy and area keep
at x times m-variations. N is changing accordingly times 1/x².
Far extern plane area at weaks:
The area keeps at m-variations. Difference-energy respectively pressure
descends to outside accordingly E/x², the force N
accordingly 1/m4 (plane area)
respectively 1/m³.(sphere
surface).
Extern elipse
area at weaks: Area varies at m-variations by
x². p and diff-E change by 1/x² times more
than at strongs (strongs: strong E keeps). N is changing accordingly 1/m³.
At weaks the
difference-energy varies with x times distances according by 1/x².
2c)
Summarize
of energy behavior of strongs/weaks
At a distance variation (plane area) to 1/x of
one original charge the force increases to x² with the approach.
At the rotation particle additional distance
ratio and angle of the rotators are changing with approach. To these the
difference force varies quadratic.
With distance variation from a rotation
particle the difference energy of this particle varies. Therefore the pressure
at the plane area or the ellipse surface will change additionally by this
factor.
The weak has to every other distance another
energy which varies by the distance variation.
2d)
Formeleinsatz
zum Energie-Vergleich
Original charge: E = p* V >> if N = *4
>> p= *8 >> average p= *4
For the plane area we have p=*4, for the
spheric energy we must take p= *8 !
Rotation system: E=p*V >> if N= *16
>> p= *32 >> average p= *16 (area don't changes)
For the plane-area at weaks we have p= *x4,
for the elipsed energy we have to take p= *x³ !
E at rotation
systems = *x5p * 1/x³m³ = *1/x²
!
At rotation particles the energy is depending
on the distances !
This may not be confused with the simultaneous
conservation of the energy of this pariticle (at each same distance).
2e)
Caution
at the masse effect
Energy E at charges is 3-dimensional >>
with more pressure p the energy E has to increase in all directions
(3-dimensional) !
Attention at more masse:
With growthing of an atomic kernel from 1 to 4
protons its force increases about 4 times. The energy also increases with times
4 !
The plane-force growed proportional to the energy !
{
Original charge: at E = p*V >> with ½ distance
there are increasing p*4 and N*4 (plane area) respectively p*8 and N*2 (sphere
surface) ! }
Minos: with ½
distance there are increasing p*16 and N*16 (plane area) respectively p*32 and
N*8 (sphere surface) ! }
With equal weaks the difference energy to
outside behaves times 1/x² (force times x-4) to x times distance
variations.
According on the radi of original charge U4 of
a 4-particle, the bigger or smaller is the energy of this particle with the
same masse and definite distance.
Simultaneous the energy is at the same
4-particle (same r of U4) to outside permanently different with additionally distance !
With the old energy-masse-relation we have to
be carefull extremly. Mostly it is not true. But within shells it can be used
if their thereby particle are all the same (same radii of 4-particles).
3)
Light
. . to 2. . . to 4. . . to 5. . . .to content . . to
the beginning
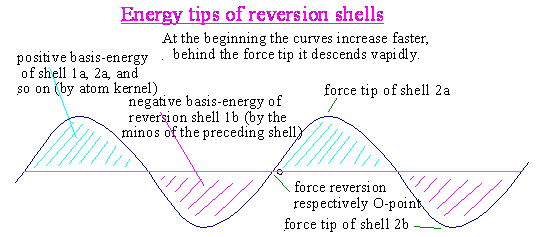
3a)
Shells,
force tips, filling
The border of every atom has a lot of
alternating plus-minus-shells.
The next following exterior shell is each
opposite directed (for ex. minus instead of plus).
Every shell begins inside at null, reaches
quickly its force tip and is running again to zero.
A shell can fill up to the force tip of the
next reversion shell !
Often it happens, that one or more negative
particles of a plus-shell is been pushed over the force tip of the bordering
exterior negative shell.
Up to this force tip the particle (minos) would be braked.
From the force tip of the reversion shell on
the minos will be accelerated to outside according the
shell energy and its own force.
Light particles are accelerated to light speed
c.
Because different waved light has different
forces and is accelerated by different shell, the light speed c varies
accordingly.
The energy of an speed
difference can be computed by the masse and this speed.
By multiplication of masse M times speed c we
get the impulse I.
The energy is computet by impulse I times this
speed c.
Thereby we get the formula E = M * c².
Energy and masse of a light-particle are still
unknown. But we could it localize up to nearing amounts (here we renounce to
that).
In no case the speed-energy of light may be
confused with the own-energy of light ! (Own-force
effect of the particle, it makes no difference how fast it is in this moment)
How fast the light will fly is additionally
depending on the accelerating shell !
Corresponding high is the light speed and
thereby the result E=M*c².
With equal own-energy of the light particle
there are possible unendless much light speeds and thereby infinity many values
of E=M*c².
3e)
Always
2 belong to the acceleration
To the acceleration force always belong 2,
either 2 repelling or 2 attracting subjects.
At the light we have shells of the atom and the
light particle for itself.
With the shells we have the first repelling
shell, which became for ex. full and lets particles run over into neighbour
shells.
3f)
Acceleration
of light in neighbour shells
The neighbour shells are reversion shells
(contrary force direction).
First the negative neighbour shell brakes the minos (here light) up to its force tip.
Behind the force tip this negative shell
accelerates this minos, because they both have the
same force direction (for ex. to c).
The next shell is more long-waved and is first
accelerating again. Behind its force tip it accelerates again.
3g)
Permanent
brake and acceleration in follow shells
This braking and re-acceleration does every
follow shell to outside.
The speed of the light particle keeps hereby
totally nearly unmodified.
The accelerating light flies from shell to
shell, whereby it is braked and accelerated permanently.
Thereby it can also be captured by a shell.
3h)
Re-capturing
respectively c-loss
Has the light-particle a clear smaller force
diameter than particles in exterior shells, it is mostly speeding without
problems through these shells.
It also can strike out such particles
, whereby it will be braked for itself.
With adding distance within the atmosphere,
fluids, glass, and so on light is becoming slowlier and increasingly caught.
The own-energy of light results of pressure p
times volume m³ of its participating original charges
E = kg/ms² * m³ = M * m/s² *m
If one would have the extern particle diameter
(force diameter), both pressure and volume of the light particle can be
computed by them (E=N*m; N is the force of the sphere surface of the particle).
The masse of a light particle results of the
force N, by which it is accelerated to c, divided by this acceleration (M = N /
m/s²).
3j)
Shell's
force structures very unprecise
By this there is necessary the force of the
light particles during the acceleration and the force structure of the
accelerating shell.
The energy respectively force of the repelling
shell can be calculated respectively isolated only very difficultly.
From both the total force N of the acceleration
to c will be calculated. In the follow there is renounced on these mathe.
4)
Quants
. . to 1. . . to 2. . . to 3. . . to 5. . . to content . . to the
beginning
The light of H-gas has 3 marked off
wave-lengths, red, blue and violet.
Every colour arises from an own shell at the
H-border.
At other atoms we find alike proportions.
Shall red light be striked out, so weaker
forces are necessary, but at violets shorter-waved more powerfuls.
Is there iron Fe melted, at the beginning it
radiates red with melting.
Is the temperature further increased, so it
increasingly radiates white light.
The red light-particles are placed further
outside and have a longer wave-length respectively force.
Therefore the red particles are delivered to
outside first.
Becomes the shell full with blue respectively
violet light, then with further take up of blue respectively violet light they
fill respective the next outside placed shells
The reds will be displaced in their own shell
increasingly.
Thereby increasingly blue and violet particles
speed to outside and cause white light with the red.
4c)
Quants ?
If iron is feeded with longer-waved particles
(for ex. only reds), then iron cannot glow white.
Is iron only yet feeded with longer-waved
particles, (no reds), so iron cannot melt.
Do you wants to melt
throug a tank with a cigarette lighter, this will fail.
The cigarette lighter provides the wrong
particles. It has too long-waved light.
In the last 2 centuries the scientists
believed, that the temperatur increases proportional with increasing quantity
of warmness. From iron on this thought wasn't more correct.
Planck delivered a theory, by which the
particles are being pushed out with shorter wave-length brokenly and harder.
4d)
Weaks
normally not quantable
Normal experiments to fit positive and negative
4-particles to larger compounds are failed up to now.
All equal directed weaks basically repel each
another.
Are coming a positive and a negative weak
together, they construct either one own big with the same force direction to
outside (for ex. 8-particle), or one of both will be turned (besides if the
force heights are extremely parting - then they could quant).
With turning only positive respectively only
negative weaks are coming out, which again repel each another.
4e)
Quanting
only in chaos or with extrem separation
A quanting of weaks of the nature is excluded
at unilateral surrounding.
Within chaos of a supernovae
suddenly positive and negative units are in confusion.
A quanting of weaks is here possible in small
bulk.
With knobs on these would mostly have more
masse in ratio to the energy, because positives and negatives are 'weaking'
each another.
Such would settle down further inside the
material, but not as light outside.
After stabilising after a chaos in every shell
there are only yet either positive or negative weaks.
A further quanting is now excluded.
Therefore in the follow will be extactly
explained, how the the nature is working without quants !
5)
Quantity and quality of minos
to
3. . . to
4. . .
to content . . to the beginning
The temperature also must be distinguished in
different qualitys.
All weaks, also the electricial current, is
subject to the same nature-laws.
With electricial current we have amperage and
votage. This applies to all weak particles.
All weaks have a force to outside.
These also can be named as temperature.
Therefore also the temperature must distinguished in quantity and quality, that is in voltage
and amperage.
5b)
Atom
bond with different shells respectively waves
The atoms border with their shells to each
another.
The one border with shells
shorter-waved, the others with shells longer-waved particles to another.
These shells hold their atoms together.
If one increases/descends their particle
quantity, their bond will be weaker/firmer.
To melt aluminium, there is
needed particles, which have at least the wave-length, which is in their bond
shells. Longer-waved particles don't separate these atoms.
To melt iron, particles are necessary, which
have a lot of shorter wave-length.
Is the wave-length too long, then iron can be
delivered yet so much temperature, it will not melt.
Is the wave-length exact correct, we only need
a minimum of energy respectively of these particles to separate iron.
5d)
Wave-lenths
and temperatures
The sun has at its border a temperatue ring of
some millionen grades of warmth, the corona.
This corona may not be imagined as a heat ring.
The particles of this corona are only
accordingly short-waved.
Normally the old physics has treated
temperature, waves and colours all the same and all confused.
Red, blue and violet colours have not
absolutely 6000 up to 10000 celvin, how this is showed for ex. by computer
monitors.
They have only the particles of this
temperature (6000-10000K) but not the quantity for this heath.
Therefore we all see this
colours with this temperature in a surrounding of a heath of 300K.
In reallity only the externe particle radius,
the wave-lenght is accordingly short at very less particles.
Also temperatures of many 100 millions of grad
within the sun so are interpretable wrong.
The weak particles round nucleon- respectively
atom kernel are accordingly shorter-waved.
5e)
Feature
variations at changed wave-length
Step by step one has to learn to build in
qualities at the temperature.
If atoms are feeded with very
short-waved, so the longer-waved will be driven out.
When these atoms ‘cool off', in no case
these shorter-waved will be extruded out of shells for normally longer-waved.
The temperature is sinking, but the features of
these atoms keep changed at some points.
Then for ex. aluminium cannot be anymore melt
by the old long wave-length.
At the reactor katastrophe of Tschernobyl
within the whole plant area lon-waved have been replaced by shorter-waved
weaks. These also cannot be driven out anymore in the future.
The accordingly changed shorter-waved features
of material will keep.
Shorter-waved particles (for ex. light) also
can settle down in shells, in which are above all longer-waved.
Because longer-waved have a bigger difference
force producing radius, there are many shorter-waved necessary to replace one
corresponding longer-waved.
Reverse this means:
Is one longer-waved electro-particle promoted or shot
(for ex. at the border of a Cu-line) into a shell further inside (shorter-waved
particles), so there were driven out respectively fired out by circustances thousands shorter-waved (for ex. light) at a single blow.
This correlates the hitherto deliver of quants ! But the single light particles itself are parted.
Electricity consists of ampeage and voltage. We
name their particles 'electros'.
The voltage is the force, which is varied with
the intern particle diameter respectively the wave-length.
The bigger the particle radius, the powerfuller
is it, and the farther outside it is placed.
Electros have a so long wave,
that they move at the outside border of the whole body, the current
line.
A transformator moves in the one circuit
shorter-waved, in the other longer-waved electros.
The longer-waved, the higher is their voltage.
The higher the voltage, the farther outside
flow these particles.
Because of the bigger distance of the higher
voltage, their force to the more inside shells is smaller.
Shorter-waved further inside, which increase
these particles which bonding the atoms, are influenced less.
The bigger the voltage,the
more power can be transported, without to burn through the line.
But attention: With 2-times voltage there
cannot be also transported the 2-times amperage !
With big alteraltions of shell further inside,
longer-waved minos are coming too far to inside.
Longer-waved are powerfuller and are being
drawn faster to inside at new compositions of shells (for ex. at nuclear
fission or alpha-decay.
Over the natural radiation of particles
permanently shorter-waved particles settle down further inside.
Now they extrude there longer-waved at the
shell border.
These are speeding to outside and can again
strike out minos from the next follow shells.
So long inside longer-waved are being driven
out, so long this particle is radiating.
Particles 4
Gases - atom-/kernel-bond - nucleons
Copyright © by
Haertel Martin, All Rights Reserved,
mailto mhaertel@naturgesetze.de
1)
Gases/atoms and their external shells
. . to 2. . . to 3. . . to 4. . . to content
1a)
Most
narrow E-radius at inert gases
Inert gases have a full last electron shell (2
electrons) respectively 3 such shells at close quarters.
This narrow last system of electron shells
delivers another plus-minus-force difference of the atom.
First all shells are narrower (descends force),
but the last shell has the double number of electrons (the negative force
increases there corresponding).
The bigger near of these 3 narrow last shells
increases the difference force at the atom border huge.
With their bigger difference energy directly
near these last electron shell system, they can take up shorter-waved particles
(have less energy).
1b)
Inert
gases have shorter-waved shells
The more norrow last shell system clearly
surpasses the opposite effect of smaller radii.
Therefore we get a higher positive force tip
behind the most extern shell.
So it gets the possibility to take up
shorter-waved particles there.
These have a smaller force reach to far
outside.
Thereby the negative force of these minos is more simple to surpass by the atom.
1c)
Inert
gases have much more and smaller shells
Behind a shell there an empty reversion shell
is coming into being with negative force.
This negative shell quickly becomes smaller to
outside and will be surpassed by the atom then positive.
In front of this reversion shell again
shorter-waved minos are daming, which produce a next
reversion shell. This will be surpassed finally again by the atom, and so on.
Because the shells at inert gases begin with
shorter-waved minos and these with their reversion
shell are being surpassed former by the atom, we get much more and smaller
shells.
Inert gases have more outside volume.
Inert gases have because of their shell
short-waveness a lot of more masse at the atom border.
These shells also reach a lot of wider to
outside.
Besides at the border they still are very
short-waved.
Heavier inert gases each are overall still
shorter-waved.
The Gravitation causes at regrowing stiff of
iron the firm atom bond.
Regrowing stiff without gravitation delivers
gasic iron with low temperature !
Fe-atoms are negative at their border negativ.
It is depending on the quantity of minos respectively the wave-length they have at their
border.
Therefore the negative border can end very
early but can reach also very far to outside.
1e)
Atom
to outside positive and negative at the same time
Electrons and protons have strong forces.
Because of bigger average distance of the
electrons and their angle against extern far reference areas it seems first,
that they could effect a smaller sum of force to outside than the kernel.
But in cause of the
quadration of the distance effect of each strong to extern
reference areas, the electrons effect by average a
bigger sum of force to outside than the kernel.
All strongs have force
reversions. All electrons have a high negative energy at their border and a
strong positive to far outside.
Because of the strong positive force to far
away, the electrons and therefore the whole atom attract all negative, which
are daming in front of the exterior electron shell.
Then at the atom border negative rings are
coming into being, which growing from border to
outside.
This atom is to near outside negative and to
far outside positive.
2)
Bond of atoms
. . to
1. . . to
3. . . to 4. . . to 5. .
. to content . . to the beginning
Electrons and protons effect by force to far
outside and realizing the basis for the gravitation force.
More atoms attract better positive.
At 0 Kelvin (0 K) atoms are more positive to
wide outside.
Additional minos weak
the positive force. Here the atom border is becoming permanetly more negative
and the negative force is be pushed always farther to outside.
Later when the border is full with negatives,
still only new arriving short-waved (have less force) are driving out
longer-waved anymore. Then contrary the negative atom border will become
smaller.
At 0K free atoms are without pressure outside.
They are 'empty'.
At 0 K free atoms are repelling each another
(positive).
At minos delivered,
the atom border is becoming more negative.
When the negative border of one free
atom is reaching the positive 2. free atom, they can
attract each another.
From a definite minos
energy on, the attraction of a nearly empty 2. atom to
the negative border of the first is greater than the repulsion between both
empty atoms.
Then both atoms bond each another.
Every atom has a bond curve.
By adding more minos,
from a definite quantity of minos energy on we get the biggest bond force (firm
condition).
By adding still more minos,
the bond force descends again, up to be fluid and finally gasiform (all repel
another).
From 0 K on up to the gas state we get a bond
curve which is depending on the minos energy.
2d)
Basis
of the aggregation condition
Once the aggregation state is depending on
which element it is.
Thereby is decisive what how many electrons and
with what radii an element has.
Corresponding above the wave-lengths of shells
at the atom border and their external border are behaving.
With 0 K the inner shells keep all untouched.
Bonded atoms, molecules, and so on are loosing
at 0 K only the surpluss of each shell.
3)
Melting points of shells
to 1. . . to
2. . . to
4. . . to 5. . . . to content . . to the beginning
3a)
Why
are gases aerially ?
Spezially with gases this has to do less.
All elements are gasiform at different
temperatures.
Why are atoms/molecules firm/fluid at more or
less temperature ?
Melting- and boiling point are depending above
all from the last electron shell, whether that has 0, 1 or 2 electrons.
3b)
Melting
point and length expanse - Pb-Ir
The product of melting-point (in K) and
length-expanse coefficient (with warmth variation) is very close quarters at
nearly all elements. The fluctuation aggregate about the
factor 2.
Is the melting point high, so the length expanse
is small and contrary.
The distance between the mid-points of 2 equal
firm atoms is comparatively high at low melting point (not always).
So lead (Pb 82e) needs at room temperature
about the 2-times space per atom how iridium (77e).
You can ask whether lead is bond very far
outside and Ir very closely. Far inside the 4-particles are shorter-waved.
Or has Pb because of its electron shell
constitution less energy to outside and isn't drawing the other atom so near ?
Ir has a big diameter of the last electron
shell and 2 electrons inside. The last but one shell has 7 electrons (uneven).
This odd electron is pushing the last shell to far outside. Therefore Ir has at
the border so much energy.
3c)
Generally
to‘only 1 electron in a shell'
Atoms with only 1 electron in the last or last
but one electron shell bond each another partly very firm (mostly very unlike
their element neighbours). They have a mighty and shorter-waved shell system of
minos. The high energy can draw the atoms very close
to each others.
With these atoms the atom has at the end of the
last electron shell a very big radius and the distance to the next atom is
comparatively very low.
When only 1 electron in the last
shell delivers the 1. force reversion, the force tip of the first
minos shells (behind last electron) is relatively low.
If the last but one has an odd electron number
and the last shell has 2 electrons, the atom builds up a mightier negative
border.
3d)
1
electron outside and full shells inside - Cu,Ag,Au
The full inside electron shells draw their
shells near to each others.
The one electron in the last shell has a big
orbit radi.
Therefore it has a lot of energy to outside,
but less against them with an odd electron number in the last but one shell and
2 electrons in the last.
Therfore the melting point and firmness is
against them some less.
The minos-shell-system is longer-waved and more
negative and can transport their special electro-minos better.
3e)
With
only 1 electron outside very easy to bind
Here the atoms have from inside the ideal
difference energy to hold at the atom border exact the negative energy to draw
extern atoms and bond them.
This negative energy is not too less (not
attracting) and not too high (repelling).
The strong force height is because of the raised
radi of the last electron exact so high, that the border can hold enough
longer-waved particles, which realize enough negative energy.
More difference energy from inside would
realize shorter-waved borders with less energy.
The sphere surface behind the last electron
shell is big enough to hold tight a big quantity of relative long-waved
4-particles.
In these shells there are gathering enough
longer-waved minos, which have a much bigger negative
force (compare Fe - Cu).
Because these atoms are at close quarters,
their gravitation effect per area unit is corresponding large.
3f)
Effects
at only 1 electron in last shell
In the case of long-waved particles relative
less masse of minos is enough to compensate the
positive force.
These atoms with only one electron in their
last shell need up to the last electron shell more space.
With the bigger electron radius the difference
force to outside increases very overproportional.
Therefore H has against He
a big positive force and a huge sphere surface to the bordering shells of
minos.
With for ex. 1,58-times
distance of an electron from the atom kernel (for ex. about H contra He) we get
for ex. the 4-times force to an outside minos.
Additional we get behind the last electron
shell the 2,5-times sphere surface and the 4-times
space.
Then H could take up much more equal minos than He.
But because of the higher shell energy, H takes
up more shorter-waved.
Therefore He will melt earlier and with less
powerful minos than H !
So atoms with only 1 electron within their last
electron shell have proportional a bigger masse and quantity of minos. With the bigger shell energy the elements are taking
up shorter-waved particles.
3g)
More
to of effects of 1 or 2 electrons in last shell
Atoms with 2 or more electrons within their
last electron shell have a corresponding smaller masse and quantity of minos. They cannot capture so much short-waved particles
(against their element neighbours) and will melt earlier. They keep more
neutral and don't effect so far negative.
The firmness of bonding is depending on the
deepness of the shells with which the atoms are binding. The ideal minos energy
would be the best (not more, not less) !
With more energy by the exterior shell (for ex.
1 electron) each force reversions is ending later, because they take up
shorterwaved minos. There the shell would need a lot
of more wideness to reach the same energy (it has to compensate more positive
energy). The border of such a free atom will become very wide and therefore
wide negative. The border of such crystal atoms will end earlies because of
their bond. These crystals effect very positive to outside.
Atoms with full shells (mostly gases) are very
narrow, have less energy and can only hold longer-waved 4-particles.
Helium can become firm, if it is not too
short-waved at its border (short-waves have less energy and need so more
particles and more space, then both atoms are too far away and repel each
another).
3h)
Result
with only 1 electron in end-shell
With only 1 electron within last or last but
one electron shell we get more shell energy and shells with shorter-waved
particles.
Mostly we reach the border to the next atom
with shorter-waved and a lot of less shells (inspite of a wider negative
border).
The wave-lenth is increasing overproportional
to the intern radius of the minos. The longer-waved
the minos at the bonding shell, the lower the melting
point. Less shell energy can hold no short-waves. There the melting point is
more could.
Do you want to melt material which is very
narrow respectively shortwaved bonded, you need at least such short-waved particles.
You cannot melt an armour
with a cigarette lighter. That lighter produces too long-waved warmth.
There bundled up laser light is a lot of
shorter and can intrude into the interstices and stretch and finally dissolve
the atom bond.
4)
Bond shells of the atom kernel
to
2. . . to 3. . . to 5. . . to 6. . . to content . . to
the beginning
4a)
Common
to protons and neutrons
Electrons have a very negative border and
effect far away strong positive (positrons contrary).
Protons also have a very negative border and
effect far away strong negative (alfas corresponding).
The circumstances of atom kernels and protons
are extrem stabil. Only under very precise circumstances they can be changed.
Neutrons are in middle near general weak
positive and repel each another.
Protons are generally in middle and far
distances strong negative and repel each another.
At big atoms protons reside at the kernel
border because of the bigger middle force against neutrons.
At protons one electron is absent. Therefore
they have a great negative surplus to middle and far outside.
At reception of one neutron the kernel and the
new neutron deliver by average overall a masse of 1% of a whole nucleon (masse
defect).
4b)
Clearance
of protons and neutrons
The nucleons are by average about 1,8 times of their diameter apart.
That correlates proportional about the average
clearance of firm atoms to each another.
At the kernel border of a big atom to every
proton borders 1 neutron (lengthwise) in square angle to the protons. The
protons are diagonal apart.
Therefore the protons have over the 1,41-times distance against each another than to their other
neutrons.
4c)
Extern
force reversion of the nuleon-/atom-kernel
Have the neutrons in the inner a more times
strong negative kernel of positrons, then exactly so many strong positive
electrons are circulating round this positron kernel.
Directly at the border of the nucleon kernel
there are shells filled with positive plusos.
Therefore this kernel effects
extremly positive at its border.
Behind this shells the
force is reversing back to strong negative to far away.
This strong negative force is holding the
electrons (high negative border) which are very positive to far away.
One big nucleon has for ex. 100 - 200 electrons
in an orbit round the kernel of 100 - 200 positrons.
These electrons have a positive force to far
away and building up positive shells filled up with negative minos
at the nucleon border.
This shell system is principially the same as
at the atom border.
The shell system begins at less than 101% of
the last electron radius.
4d)
Bond
shells of the nucleons
The neutron is very positive to far away
(electrons have the radius, positrons are in the point).
The neutron attracts all weak negatives from
outside and fills up its shells at its border.
The maximal difference force between rotating
electron and standing positron is for ex. at a radius of 101,8%
of the last electron and has 6,66 % force of one electron.
At a radius of 1,1-times
(1,8-times) the force would be about 0,26% (0,003%).
To be filled up the nucleon needs for ex. 1030
weak negative 4-particles.
By filling up new empty negative reversion shells
are produced.
After the first reversion shell 1b new arriving
minos are daming in front of this 1b-shell. They fill
up the shell 2a. These particles inside 2a produce with their negative energy
the reversion shell 2b, then daming for 3a, and so on.
Because of these arriving minos
the positive difference force at the neutron border is descending quicklier.
The electrons are producing positive shells
which have been filled up negative under production of reversion shells.
We name these shells with negative minos the bond shells of the nucleons.
4e)
Bond
curve of the nucleons
The ‘gasiform' is depending above all how
many minos energy the nucleon has at its border.
With null minos masse
they repel each another strongly.
With adding minos,
repulsion descends increasingly, up to mutual attraction.
At a definite minos
energy there could be spoken of a firm state or of the highest bond force also
at nucleons.
By adding still more minos,
the bond force will descend again, up to all repel each another and become
gasiform.
From the weakest bond on up to the gasiform we
get a bond curve which is depending on the minos
energy (compare 0 K at full shells at the atom border).
4f)
Neutrons
are also no gases
Neutrons are a little bit more aerially and attract
therefore mor negative weaks.
Would have the nucleon at its border very many
electrons within the last shell, so the nuclen would be intern the most narrow.
Then it would surround itself with so much
negative bond masse, that all nucleons would be
aerially.
Actually neutrons are delivering no pure
aerially behavior (react with protons like F with H).
Neutrons disgust each another in the normal
state.
Neutron are negative to outside at middle
distance, then at farther positive !
The strong parts of the neutron (positrons,
electrons) would have a positive force to outside at enough distance.
Gases have a full exterior electron shell at
the atom border.
Therefore all last electrons are attracted very
near to the last but one shell.
Within the neutron this also would be the same.
At loss of electrons the proton reacts a little
bit other than the atom at electron capturing.
At the proton there is missing one electron.
But it has the same positive force from the nucleon kernel.
4h)
Negative
Soup of the nucleon kernel
The negative soup of the nucleon kernel which
is clinging the protons together, is negative.
It weaks the positive kernel effect
over huge distances to the most exterior electron border (*105) only minimal
because it is weak.
Consequences to the bond shells are therefore
inconsiderable.
5)
Proton/Neutron - which last electron ?
to 3. . . to 4. . . to 6. . . to 7. . . to content . . to the beginning
5a)
Loss
of electrons - - bond shell
The causes for the force of the bond shell are
primary by the number of electrons of the nucleon..
A proton has one electron less.
but: It isn’t so, that the bond shell, with
1 electron less, can attract more minos in this quantity.
The bond shell even looses masse.
To break down this there are 2 electron examples.
Either the neutron looses at electron loss its
last electron shell or not.
5b)
How
many electrons has a nucleon in exterior shell ?
A neutron could have for ex. 6 electrons within
a full exterior interior electron shell and the proton exact 5. About exactly this
effect is obtained by follow:
A proton could have within the exterior
interior electron shell only 1 electron and the neutron exaxtly 2.
So the exterior electrons of the neutron would
be maximal apart from another and have only less higher
radii (force of the neutron kernel doesn’t change).
5c) Differenz der ersten Schalen bei Neutronen/Proton
Wir nehmen nun an, dass ein Proton eine volle äusserste Schale hat.
Das Neutron hat eine Schale mehr, in der nur 1 Elektron kreist.
Die 1. Kraftumkehrung des Neutrons wäre deutlich weiter aussen als beim Proton.
Allerdings hat das Neutron aufgrund soviel Positronen wie Elektronen eine geringere starke Kraftdifferenz von innen.
Das Proton wäre früher positiv.
5d) Proton hat eine E-Schale weniger ?
Die Schalen dieses Neutrons wären demnach theoretisch weniger und langwelliger und gingen räumlich viel weniger nach aussen als beim Proton.
Das Neutron wäre dann wie ein Au-Atom.
Die Mächtigkeit dieser Schalen des Neutrons wäre demnach kleiner als beim Proton.
Allerdings ist der negative Kraftanteil des Protons aufgrund des fehlenden negativen Elektrons niedriger.
Beim Elektronenausklinken kommen aber keine kurzwelligeren Minos zum Proton.
Dann nisten sich die langwelligen des Neutrons um das innen engere Proton.
Das Proton kann dann im Verhältnis nicht die Masse halten, wie entsprechende Atome am Atomrand.
Das Proton zieht Minos an, bis es voll ist.
5e) Letzte E-Schale mit 1 bzw. 2 Elektronen
Wenn das Neutron in seiner letzten Elektronenschale voll ist, so ist es extrem eng.
Die 1. Kraftumkehrung kommt nun viel näher (ein Elektron beim Proton in letzter Schale):
Die positive Kraft auf Schale 1 beim Neutron ist im Verhältnis viel höher.
Die Kraftspitze in Schale 1 ist entsprechend hoch.
Die Schalen könnten weniger dieser Langwelligen aufnehmen.
Man bräuchte viel mehr Raum um eine ähnliche Menge an Bindemasse unterzubringen.
Das Neutron müsste leichter sein, als es in Wirklichkeit ist.
5f)
Schale
1 ohne letzte E-Schale
Wenn das Neutron in seiner letzten Elektronenschale nur 1 Elektron hat, verliert es diese Schale.
Die 1. Kraftumkehrung kommt nun viel näher (kein Elektron beim Proton in letzter Schale):
Die ‘vorletzte’ (neue letzte) Elektronen-Schale wirkt höher.
Die negative Kraft auf Schale 1 ist im Verhältnis viel höher.
Die Kraftspitze in Schale 1 ist entsprechend niedrig.
Die 2. Kraftumkehrung kommt im Verhältnis zur 1. später. Man braucht mehr Raum um eine ähnliche Menge an Bindemasse unterzubringen.
Die Nukleonen haben so mehr Abstand voneinander, obwohl das Proton innen enger ist.
Beim Elektronenverlust eines Neutrons verliert das Neutron eine Masse an Schwachen, welche etwa 0,087% der Gesamtmasse eines Neutrons ausmacht.
Macht die Masse der Schalen um das Nukleon etwa 5% der Gesamtmasse des Nukleons aus, so verliert das Neutron beim Elektronenverlust weniger als 2% dieses Schaleninhalts.
Bei Aufnahme von Neutronen durch den Atomkern verliert das Neutron durchschnittlich 1% der Geamtmasse bzw. nach obiger 5%-Annahme etwa 20% seiner äusseren Schalenmasse.
Wenn ein Atomkern ein Nukleon aufnimmt, so ist es ähnlich, wie wenn 2H und 1O zu Wasser verbrennen.
Wenn ein Neutron am Rand des Atomkerns tanzen würde, so hätte es kaum Massenverluste.
5h)
Massendifferenz
von Proton/Neutron unproblematisch
Neutronen haben innen einen grösseren Durchmesser als die Protonen.
Die Neutronen-Bindeschalen haben aber nur minimal mehr Kapazität (verliert bei Elektronenaustritt nur 0,087% der Nukleonenmasse)
Da die Nukleonen um etwa ihren 1,8-fachen Durchmesser auseinander sind, machen diese Unterschiede nichts aus.
Zwischen den Kraftumkehrungen der Bindeschale ist bei Proton und Neutron nur wenig Unterschied.
5i)
Schale
1 des Atomkerns als Bindematerial
Sowohl Neutron als auch Proton wären ab der 1. Kraftumkehrung nach aussen positiv und ziehen schwache Negative an (wegen höherem Elektronen-Radius).
Sie füllen diese ihre Schalen mit Negativen voll, welche entsprechende Umkehrschalen erzeugen.
Sie ziehen die sonst hier positiven Nukleonen zusammen und halten sie gleichzeitig auf Abstand 1,8.
6)
Kraft und
Aggregatszustand des Kerns – Elektronenaustritt
. . to 4. . . to 5. . . to 7. . . to 8. . . to content . . to
the beginning
6a) Kraftdifferenz von Proton und Neutron weiter weg
Der Unterschied zwischen Neutron und Proton ist derjenige, dass das Neutron keinen Überschuss an starken Urladungen hat.
Die schwache Kraft fällt nach aussen schneller als die Starke. Bei 1/10³-fachem Abstand fällt die starke Kraft auf 1/106, wobei die Schwache auf etwa 1/108 bis 1/109 fällt.
Daher wirkt das Proton auf grössere Entfernung wieder stark positiv, wobei das Neutron lange schwach negativ bleibt.
Bei zB 6 Elektronen in äusserster Atom-Schale wäre bei 6 starken Positiven (Kraft +6) im Nukleon-Zentrum die starke Kraft an der Stelle eines Elektrons bis +4,5 stark.
6b)
Schalenumkehrungen
des Neutrons
Da sowohl die Positronen, aber auch das ganze Neutron am Rand eine extrem dichte Masse aus schwachen Negativen (Minos) aufweisen, erzeugen sie immer wieder Umkehrschalen.
Vor diesen stauen sich wieder Minos und erzeugen wieder negative Umkehrschalen, usw.
Sowohl Neutron als auch Proton wirken am Rand in den entscheidenden Bereichen immer wieder negativ.
Sie ziehen am Rand alles Positive an (Protonen, Plusos).
Ein Neutron würde am liebsten ein Positron aufnehmen.
Aber alle grossen 'Positiven' sind am Rand auch negativ.
Es müssen die Wellenlängen am Rand zueinander passen, wenn Neutronen bzw. Protonen aufgenommen werden sollen.
Es entscheidet nicht die Menge, da der Atom- bzw. Nukleonenrand sowieso immer voll ist.
6d)
Aggregatszustand
der Nukleonen
Nun sind die Nukleonen im Atomkern aber gegenseitig weder fest noch gasförmig.
Am ehesten könnte man sie noch als flüssig bezeichnen.
In gewisser Hinsicht ähneln Atomkerne gasförmigen Grossmolekülen.
Bei Molekülen kommt es darauf an, ob die Elektronenschalen 1 oder 2 Elektronen haben.
Chemiker könnten zum Thema Proton/Neutron sicher Kombinationen aus der Molekülwelt finden, welche präzise zum Elektronenrand passen.
Vielleicht wäre ein Neutron auch leicht mit einem Quecksilber- oder Bleiatom vergleichbar (80/82 Elektronen).
6e)
Hg
- Au und ihre Elektronen
Bei Verlust eines Elektrons würde Quecksilber schwache negative Masse abgeben, da das übrigbleibende Elektron der 6s-Schale nun weiter aussen rotiert.
Quecksilber hat einen kleineren Radius der letzten Elektronenschale, da es gegenüber Gold (79 Elektronen) 2 Elektronen aussen hat.
Dieses wird verursacht, weil Hg ein Proton mehr und damit eine höhere Kraft als Au hat. Beide Elektronen der 6s-Schale gehen mit reduziertem Radius auf maximalen Abstand zueinander.
6f) Letztes Nukleonen-Elektron: Allgemeines zur Schale
Ein oder mehr Elektronen rotieren im Nukleon in dessen
äusserster (letzter) Schale Þ
Die starke positive Kraft aus dem Inneren hält das letzte Elektron gerade noch in der äussersten Nukleonenschale.
Den Bereich der positiven Kraft im Nukleon nennt man innere Plus-Schale des Nukleone.
Den Bereich der negativen Kraft der letzten Elektronenschale im Nukleon nennt man letzte innere Minus-Schale des Nukleons.
Schwache können fast nicht zur Elektronenschale durchdringen, es sei denn sie sind eng genug.
Der Nukleoneninnenraum bzw. Kern ist sehr geschützt.
6g)
Äusserstes
Elektron extrem instabil
Ausserhalb der letzten Schale hat das Nukleon eine Kraftumkehrung von Minus auf Plus.
Jedes Elektron hat einen positiven Rand.
Negative Schalen mit Kurzreichweite können diesen Rand noch erwischen, sind aber weiter weg zu schwach, um das Elektron negativ abzustossen.
Das äusserste Elektron ist so instabil, dass es bei sehr wenig von aussen eingreifender negativer Kraft kurzer Reichweite das Nukleon verlässt und mit Abstand um den ganzen Atomkern kreist.
Die 1. äussere Kraftumkehrung des Neutrons ist scheinbar extrem nah am letzten Elektron.
6h)
Elektronenausklinken
mit mittleren Wellen
Beta-Minus-Reaktionen kommen in der Natur unter bestimmten Bedingungen laufend vor.
Unter welchen Bedingungen verliert ein Neutron das äussere Elektron ?
Kommen zum Atomkern kurzwellige Minos, welche die inneren Schalen des Nukleons negativer machen, so ziehen diese das äusserste innere Elektron nach aussen.
Sind die Minos zu kurzwellig, so erhöhen sie nur die Abstände der Schalen und stabilisieren das letzte Elektron.
Man braucht also Minos mittlerer Wellenlänge um aus Neutronen Protonen zu machen.
7)
Force and mass at nucleon’s border
. . to 4. . . to 5. . . to 6. . . to 8. . . to content . . to
the beginning
7a)
Second
force inversion at nucleon’s border, proton
With for ex. 6 electrons within extremity atom shell
and 6 strong positives (force +6) within the nucleon center the strong force at
the place of an electron would be strong about +4,5.
Is an electron
(proton) missing, above force only rises to just under +5.
To outside the negative force subsides up to a
newly force inversion.
There the exterior plus shell of the nucleon is
beginning.
The force inversion begins at the proton a
little bit more inside and ends aome later.
The more electrons, the smaller becomes the
effect of one missing electron.
7b)
Parameter
to the masse of the atom kernel
One atom kernel can hold a nultiple number of minos than the minos shell of the atom.
Every nucleon has inside from 80 up to 200
electrons.
About the number of nucleons the force could be
higher against the atomic border additional to the distance effect.
Besides this the wave lengths are shorter at
the kernel border. This enables once more masse.
7c)
Masse
at the kernel border
The spheric areas of the shells at the atom
border are for ex. 10 billion times more than this at the nucleon border.
Ther we also find a 10 billion times bigger
strong force (with for ex. each 80 electrons at atom/nucleon).
With the same electron number so a nucleon
might have more masse at the border only about the shorter wave-lengths.
But the wave lenghts could also be shorter up
to 100.000 times.
8)
Particles 5 - Others
to 4. . . to 5. . . to 6. . . to 7. . . to content . . to the beginning
8a)
Energy
and force proportional
With 4 times proton number there is at same
distance the 4 times force to outside, but only the 4 times pressure.
At 4 times pressure there also is only the 4
times energy !
Force advantage takes place by low pressure.
Force and energy can go proportional, insteat
of 4/8 !
At 8-times proton number there is the 8-times
of energy, force and pressure to outside.
Area and distance haven*t changed thereby.
8b)
Slipping
through barrier layers
The difference of minos
shells between proton and neutron is the smaller the more electrons are
rotating at the border of the nucleon’s kernel.
The more rotate, the ‚smaller’
those negative particles had to be, which want to slip through this electron
belt into the inside space of the nucleon.
Only particles from a special impuls relative
to their force effect can intrude into thisinside space. with.
The same is valid for all minos
and all other strongs and weaks (whole atoms).
At atoms these particles are corresponding
bigger, at nucleons accordingly smaller.
Minos within the minos
shell react with arriving plusos (positive weaks).
The positives will be revolved and conversed
into minos.
Plusos and minos react
so long with each another, up to only minos are remaining.
8d)
Electrons
don’t jump between nucleons
One electron can bond 2 protons to itself !
One original charge can let round maximal 3
contrary charged original charges round itself.
3 are relative instabil, because from outside
are low forces enough to deduct an original charge.
At nucleons we have wholly other forces ratio,
because one proton is missing a whole strong negative original charge.
One neutron can bond 2 protons to itself
(Helium with 3 nucleons) These are maximal distanced
to each another.
Electrons don’t jump between nucleons
back and forth.
Inside the neutron one caught electron round
more than inside the proton.
The electron rounds in the border sector of the
neutron (for ex. 100.000 times faster than wihin the external electron shell of
the atom).
At the nucleons there are protons only at the
border of the atomic kernel, because they disgust each another.
At the proton there is missing 1 electron.